 |
 |
Rivaroxaban is an oral, direct factor Xa inhibitor for which the European licensing agency gave a Positive Opinion in July 2008 for the prevention of venous thromboembolism (VTE) in patients undergoing major orthopaedic surgery of the lower limbs. We have previously reviewed this in an “On the Horizon” bulletin. There is interest in this drug as it is an oral agent that does not seem to require the anticoagulation monitoring associated with current regimens based on warfarin and heparin. Rivaroxaban is in the 17th wave of NICE appraisals.
Action
- Should efficacy and safety data prove favourable for oral rivaroxaban, and depending on cost, the drug might be particularly appropriate for those patients undergoing extended thromboprophylaxis after hip surgery.
- There will be no necessity to monitor patients for heparin-induced thrombocytopenia and a reduction in at-home nurse visits may be possible. Hence, staff capacity may be released.
- Area prescribing committees will need to balance the possible increase in drug costs with rivaroxaban versus the possible benefits that may accrue.
- A cautious introduction may be prudent, balancing benefits and risks, until the adverse event profile is better known.
What is the background to this?
The Global Orthopaedic Registry indicates that, in almost 15,000 patients, the cumulative incidence of venous thromboembolism (VTE) within three months of surgery was 1.7% after total hip replacement (THR) and 2.3% in total knee replacement (TKR) patients.
NICE guidance recommends low molecular weight heparin (LMWH) or fondaparinux, in addition to mechanical prophylaxis, for patients at increased risk of VTE who are undergoing orthopaedic surgery. Treatment should continue for four weeks after hip fracture surgery. Implementation of this guideline could result in cost savings due to a reduction in the costs associated with treating VTE (approximately £11,000/100,000 population).
Further information about VTE is available on the cardiovascular floor of NPCi.
What do these studies claim?
Rivaroxaban has been compared to daily subcutaneous enoxaparin in three phase III, double-blind, double-dummy, randomised trials in adults (the RECORD1–3 series). Data from RECORD4 are available only as a press release and a twice daily regimen of enoxaparin was used (as licensed in the USA), so will not be considered further here.
Oral rivaroxaban was more effective than subcutaneous enoxaparin in reducing the primary outcome, a composite end-point of any deep vein thrombosis, non-fatal pulmonary embolism, or death from any cause (see table). The primary safety outcome was major bleeding – the definition of which excluded surgical-site bleeding, unless it resulted in re-operation or death. Therefore, the incidence of major bleeding in the trials was low and, unsurprisingly, there was no difference detected between rivaroxaban and enoxaparin for this outcome (see table).
Plasma alanine aminotransferase levels greater than three times the upper limit of normal were seen in 2% or less of patients on rivaroxaban and in up to 5% of enoxaparin patients. These resolved by the end of follow-up.
Reactivation of coagulation after the end of anticoagulant therapy can occur and may manifest as an increase in cardiovascular (CV) events [1]. A small number of patients in the trials experienced CV adverse events after withdrawal of prophylaxis.
How does this relate to other studies?
Dabigatran, an oral drug for VTE prophylaxis after major orthopaedic surgery, has recently been launched. In trials, it was found to be non-inferior to subcutaneous enoxaparin [2, 3]. There are no head-to-head studies of either dabigatran or fondaparinux with rivaroxaban. The Scottish Medicines Consortium has reviewed the evidence for dabigatran and has advised that it has a place for the primary prevention of VTE in adult patients who have undergone elective total hip replacement or total knee replacement surgery. NICE have recently issued draft guidance on the role of dabigatran.
So what?
An oral agent for VTE prophylaxis has the potential to increase patient compliance and release nurse time from either administering injections or teaching patients to self-inject. Clinical trials of rivaroxaban for other indications are currently recruiting. If, in the future, rivaroxaban receives a marketing authorisation for long-term conditions such as prevention of stroke in patients with atrial fibrillation, this will have implications for primary care. Rivaroxaban produces a predictable anti-coagulant response which does not need laboratory monitoring, unlike warfarin [4].
The trials used a composite end-point. Although this is recommended by the European Medicines Agency to meet licensing requirements, this approach may mislead if the components are of widely differing importance to patients and the size of the effect differs markedly across components.
Of note is the unequal duration of prophylaxis in RECORD2. Extended prophylaxis with rivaroxaban was compared with a short regimen of enoxaparin, followed by placebo.
Study details
Pharmaceutical companies sponsored all the studies. Exclusions included patients at high risk of bleeding, substantial liver disease and creatinine clearance less than 30ml/minute. Rivaroxaban was initiated 6 to 8 hours after wound closure. Enoxaparin was given 12 hours before surgery and re-started 6 to 8 hours after wound closure. Thereafter, doses were given every 24 hours. All patients were assessed using bilateral venography.
The trials were designed as non-inferiority studies. If non-inferiority was demonstrated in the per-protocol population, a second analysis was undertaken to determine if rivaroxaban was superior in the modified intention-to-treat population (patients who had undergone planned surgery, taken a study drug and undergone adequate assessment for thromboembolism).
The number of patients randomised was calculated to retain the power of the study assuming 25% of patients would not have valid venograms. In RECORD2, 28% of the rivaroxaban and 27% of enoxaparin patients did not have valid venograms. The authors undertook sensitivity analysis which indicated that this lack of venograms did not affect the power of the study or bias the outcomes.
See table for further details.
References
1. Lassen MR, Ageno W, Borris LC et al. Rivaroxaban versus Enoxaparin for Thromboprophylaxis after Total Knee Arthroplasty. N Engl J Med 2008; 358:2776-86
2. Eriksson B I, Dahl OE, Rosencher N et al for the Re-Model study group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. Journal of Thrombosis and Haemostasis 2007; 5:2178-85
3. Eriksson B I, Dahl OE, Rosencher N et al for the RE-NOVATE study group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949-56
4. Eikelboom JW and Weitz JI. Selective factor Xa inhibition for thromboprophylaxis. Lancet 2008; 372:6-8
Table- Summary of key results from the RECORD 1–3 studies
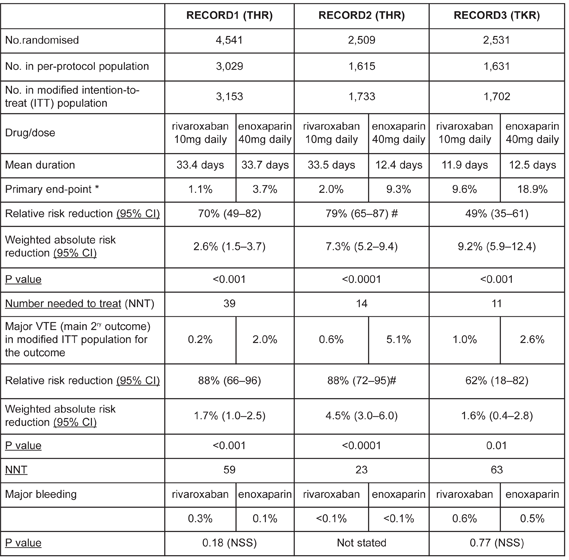 |
* Composite of any deep vein thrombosis, non-fatal pulmonary embolism, or death from any cause as used in modified intention-to-treat population
N.B. The patient populations differ slightly for each trial outcome
# calculated (not quoted in paper)
NSS- Not statistically significant
Feedback
Please comment on this blog in the NPCi discussion rooms, or using our feedback form
